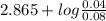Chemistry, 10.12.2019 05:31 jeffcarpenter
Calculate the ph of a solution prepared by mixing 0.080 0 mol of chloroacetic acid plus 0.040 0 mol of sodium chloroacetate in 1.00 l of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Calculate the ph of a solution prepared by mixing 0.080 0 mol of chloroacetic acid plus 0.040 0 mol...
Questions



Computers and Technology, 22.08.2020 01:01



English, 22.08.2020 01:01


English, 22.08.2020 01:01

Mathematics, 22.08.2020 01:01


Mathematics, 22.08.2020 01:01

Mathematics, 22.08.2020 01:01

History, 22.08.2020 01:01

Computers and Technology, 22.08.2020 01:01




Mathematics, 22.08.2020 01:01

Mathematics, 22.08.2020 01:01

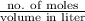
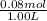
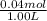
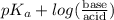
![pK_{a} + log{\frac{[salt]}{[acid]}}](/tpl/images/0411/2933/58c9c.png)