
Acertain dose of tums contains 750.0 mg of calcium carbonate, caco3, in each tablet. in this problem, you will follow the steps to calculate how many grams of stomach acid, hcl, are neutralized by one tablet of tums. balance the chemical equation for the reaction of hcl with caco3. chemical equation: hcl + caco_{3} -> cacl_{2} + co_{2} + h_{2}o hcl+caco3⟶cacl2+co2+h2o
using the balanced equation, determine how many moles of hclhcl will react with 1 mol1 mol of caco3.caco3.
moles of hcl: hcl:
calculate how many moles 750.0 mg750.0 mg caco3caco3 is.
750.0 mg caco3=750.0 mg caco3= caco3
using your answers from the first two questions, calculate how many moles of hclhcl are neutralized when 750.0 mg750.0 mg caco3caco3 reacts.
moles of hcl: hcl:
convert the number of moles of hclhcl that you found in the previous question to grams of hcl. hcl. your answer is the amount (in grams) of hclhcl neutralized by one tablet of tums.
mass of hclhcl:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1



Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Acertain dose of tums contains 750.0 mg of calcium carbonate, caco3, in each tablet. in this problem...
Questions


Computers and Technology, 17.03.2022 15:10

Mathematics, 17.03.2022 15:10

Mathematics, 17.03.2022 15:10



Chemistry, 17.03.2022 15:10

History, 17.03.2022 15:10


Mathematics, 17.03.2022 15:10


Chemistry, 17.03.2022 15:10

Mathematics, 17.03.2022 15:10


Mathematics, 17.03.2022 15:10

Mathematics, 17.03.2022 15:10

World Languages, 17.03.2022 15:10



Biology, 17.03.2022 15:20

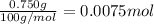
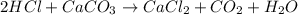
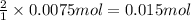 of HCl.
of HCl.

