Chemistry, 13.12.2019 00:31 JohnJamesPaksitani
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction 2h202(g) 2h20(g)+02(g) in a particular experiment, 1.75 moles of hy02 were placed in a 25-l reaction chamber at 307ec after equilibrium was reached, 1.20 moles of h202 remained what is ke for the reaction?
a. 2.4×10^-3
b. 2.0×10^-4
c. 5.5×10^-3
d. 2.3×10^-2

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

You know the right answer?
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction 2h2...
Questions





Computers and Technology, 17.01.2020 19:31

Computers and Technology, 17.01.2020 19:31

Social Studies, 17.01.2020 19:31




Geography, 17.01.2020 19:31









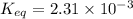
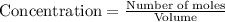
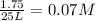
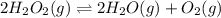
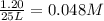
![[H_2O]=2x=2\times 0.011 M= 0.022 M](/tpl/images/0416/0677/428c7.png)
![[O_2]=x=0.011 M](/tpl/images/0416/0677/5d441.png)
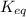 for the above reaction follows:
for the above reaction follows:![K_{eq}=\frac{[H_2O]^2\times [O_2]}{ [H_2O_2]^2}](/tpl/images/0416/0677/4f6fe.png)