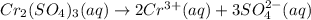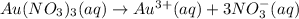Chemistry, 13.12.2019 06:31 robertss403
Iwant to use a galvanic cell to power a 60-watt light bulb. complete the following steps to determine how long the galvanic cell will power the light bulb before running out.
a.) the galvanic cell uses the following solutions: 0.81 l 1.5 m cr2(so4)3 solution and 1.2 l 0.81 m au(no3)3 solution. write down the balanced chemical equations of the dissolution of cr2(so4)3and au(no3)3 into ions in water.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Iwant to use a galvanic cell to power a 60-watt light bulb. complete the following steps to determin...
Questions


Computers and Technology, 06.11.2019 23:31


Computers and Technology, 06.11.2019 23:31



History, 06.11.2019 23:31


Computers and Technology, 06.11.2019 23:31




Mathematics, 06.11.2019 23:31




Mathematics, 06.11.2019 23:31