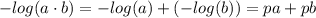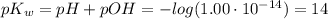Chemistry, 17.12.2019 02:31 Jasmineemarieee
The ph of a solution is the negative logarithm of the molar concentration of hydronium ion, that is, ph=−log[h3o+] in neutral solutions at 25 ∘c, [h3o+]=10−7 m and ph=7. as [h3o+] increases, ph decreases, so acidic solutions have a ph of less than 7. basic solutions have a ph greater than 7. the hydroxide and hydronium ion concentrations are related by the the ion-product constant of water, kw , as follows: kw=1.0×10−14=[h3o+][oh−] in the same way as the ph, we can define the poh as poh=−log[oh−]. it follows from the kw expression that ph+poh=14.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 14:00
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2
You know the right answer?
The ph of a solution is the negative logarithm of the molar concentration of hydronium ion, that is,...
Questions

Chemistry, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00


History, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Business, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00

Social Studies, 17.11.2020 01:00


Physics, 17.11.2020 01:00

English, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00

Biology, 17.11.2020 01:00

![pH = -log[H^+]](/tpl/images/0421/6601/7d119.png)
![pH = -log[H^+], pK_a = -log(K_a)](/tpl/images/0421/6601/29e90.png) etc.
etc.![[H_3O^+] = [OH^-] = 1.00\cdot 10^{-7} M](/tpl/images/0421/6601/e5313.png)
![pH = -log[H_3O^+] = -log(1.00\cdot 10^{-7}) = 7.00](/tpl/images/0421/6601/b738b.png)
![[H_3O^+] =2.00\cdot 10^{-7} M](/tpl/images/0421/6601/25e55.png)
![pH = -log[H_3O^+] = -log(2.00\cdot 10^{-7}) = 6.70](/tpl/images/0421/6601/deab9.png)
![K_w=[H_3O^+][OH^-]](/tpl/images/0421/6601/16faa.png)
![K_w=[H_3O^+][OH^-]=1.00\cdot10^{-14}](/tpl/images/0421/6601/58549.png)
![-log(K_w)=-log([H_3O^+][OH^-])](/tpl/images/0421/6601/4aed2.png)