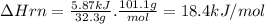Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g of kno3 is dissolved in 243 g of water at 23.00 °c. kno3(s)+h2o(aq) > koh(aq)+hno3(aq)the temperature of the resulting solution decreases to 17.90 °c. assume the resulting solution has the same specific heat as water, 4.184 j/(g·°c), and that there is negligible heat loss to the surroundings.1. how much heat was released by the solution? 2. what is the enthalpy of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g...
Questions













Computers and Technology, 11.12.2019 19:31