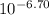Chemistry, 17.12.2019 04:31 Trishamw04
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated with 0.10 m naoh. after a total of 25.0 ml of naoh was added, the resulting solution has a ph of 6.70.
after a total of 50.0 ml of naoh was added, the ph of the solution was 8.00.
determine the values of ka1 and ka2 for the diprotic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated...
Questions

Mathematics, 13.08.2021 02:30


Law, 13.08.2021 02:30


Mathematics, 13.08.2021 02:40







Mathematics, 13.08.2021 02:40

Mathematics, 13.08.2021 02:40



Computers and Technology, 13.08.2021 02:40




Computers and Technology, 13.08.2021 02:40