Using vsepr theory, which of these molecules does not have a trigonal planar shape?
a) ammoni...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2


Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Questions

Mathematics, 08.02.2021 20:50

Mathematics, 08.02.2021 20:50




Mathematics, 08.02.2021 20:50


Biology, 08.02.2021 20:50


Arts, 08.02.2021 20:50


Mathematics, 08.02.2021 20:50


Computers and Technology, 08.02.2021 20:50


Mathematics, 08.02.2021 20:50




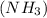 has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide
has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide 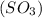 has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.
has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.


