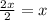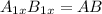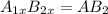In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes is 1: 1: 2. what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy half of the tetrahedral holes? ab a3b ab2 a2b what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the tetrahedral holes? ab a2b ab2 a3b

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrah...
Questions



Arts, 03.07.2019 22:20


History, 03.07.2019 22:20

Mathematics, 03.07.2019 22:20

Mathematics, 03.07.2019 22:20

Mathematics, 03.07.2019 22:20

Mathematics, 03.07.2019 22:20


History, 03.07.2019 22:20



Mathematics, 03.07.2019 22:20

History, 03.07.2019 22:20


Mathematics, 03.07.2019 22:20


History, 03.07.2019 22:20