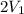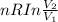Chemistry, 20.12.2019 03:31 arifkarimi9214
Calculate the change in the entropy of the system and also the change in the entropy of the surroundings, and the resulting total change in entropy, when a sample of nitrogen gas of mass 14 g at 298 k and 1.00 bar doubles its volume in (a) an isothermal reversible expansion, (b) an isothermal irreversible expansion against pext = 0, and (c) an adiabatic reversible expansion.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Calculate the change in the entropy of the system and also the change in the entropy of the surround...
Questions





Mathematics, 28.09.2019 14:30

Chemistry, 28.09.2019 14:30

History, 28.09.2019 14:30


History, 28.09.2019 14:30

Geography, 28.09.2019 14:30

Chemistry, 28.09.2019 14:30




Mathematics, 28.09.2019 14:30

History, 28.09.2019 14:30


History, 28.09.2019 14:30

Chemistry, 28.09.2019 14:30

Mathematics, 28.09.2019 14:30

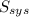 = 2.881 J/K; Δ
= 2.881 J/K; Δ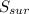 = -2.881 J/K; total change in entropy = 0
= -2.881 J/K; total change in entropy = 0 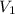
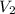 =
=