Chemistry, 20.12.2019 03:31 dukkchild666
Avolume of 50.0 ml of 0.400 m hbr at 24.35°c is added to 50.0 ml of 0.400 m naoh, also at 24.35°c. after the reaction, the final temperature is 27.06°c. calculate the enthalpy change, h, per mole of hbr (in kj) for the reaction. heat specific capacity of the solution is 4.184 j/oc, density of the solution is 1.0 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Avolume of 50.0 ml of 0.400 m hbr at 24.35°c is added to 50.0 ml of 0.400 m naoh, also at 24.35°c. a...
Questions


History, 21.06.2019 17:00





English, 21.06.2019 17:00



English, 21.06.2019 17:00


English, 21.06.2019 17:00




Mathematics, 21.06.2019 17:00


English, 21.06.2019 17:00


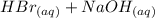 ⇒
⇒