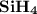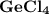1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
use the octet rule to predict the formula of the compound that would form between silicon and hydrogen , if the molecule contains only one silicon atom and only single bonds are formed.
formula:
2. the element germanium would be expected to covalent bond(s) in order to obey the octet rule. use the octet rule to predict the formula of the compound that would form between germanium and chlorine , if the molecule contains only one germanium atom and only single bonds are formed.
formula:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
<...
<...
Questions




Mathematics, 26.06.2019 12:00

Mathematics, 26.06.2019 12:00




Mathematics, 26.06.2019 12:00





Geography, 26.06.2019 12:00



Mathematics, 26.06.2019 12:00



Geography, 26.06.2019 12:00