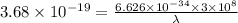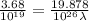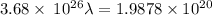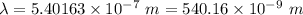Chemistry, 24.12.2019 21:31 juansantos7b
Aphoton with 2.3 ev of energy can eject an electron from potassium. what is the corresponding wavelength of this type of light? answer in nm. 1 ev = 1.60 x 10-19 j speed of light = 3.0 x 108 m/s planck's constant = 6.626 x 10-34 js socratic.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1


You know the right answer?
Aphoton with 2.3 ev of energy can eject an electron from potassium. what is the corresponding wavele...
Questions


Mathematics, 11.12.2020 02:30

Mathematics, 11.12.2020 02:30

Arts, 11.12.2020 02:30

Mathematics, 11.12.2020 02:30


History, 11.12.2020 02:30

Mathematics, 11.12.2020 02:30


Mathematics, 11.12.2020 02:30

Mathematics, 11.12.2020 02:30


Physics, 11.12.2020 02:30

Mathematics, 11.12.2020 02:30



Mathematics, 11.12.2020 02:30


Mathematics, 11.12.2020 02:30

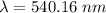
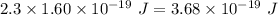
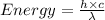
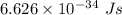
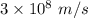
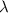 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded