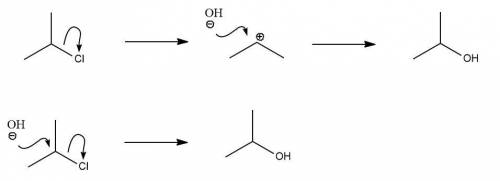
The reaction of 2-chloropropane with sodium hydroxide can occur via both sn1 and sn2 mechanisms.
a) draw arrow pushing schematics for each sn1 and sn2 mechanism for this reaction.
b) identify the rate determining step for each of the mechanisms you drew in q2.
how exactly do i do this?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3

Chemistry, 23.06.2019 23:00
For a particular first-order reaction, it takes 24 minutes for the concentration of the reactant to decrease to 25% of its initial value. what is the value for rate constant (in s-1) for the reaction?
Answers: 2
You know the right answer?
The reaction of 2-chloropropane with sodium hydroxide can occur via both sn1 and sn2 mechanisms.
Questions

Computers and Technology, 06.12.2021 22:10

Mathematics, 06.12.2021 22:10



History, 06.12.2021 22:10



Mathematics, 06.12.2021 22:10

Health, 06.12.2021 22:10

History, 06.12.2021 22:10

Mathematics, 06.12.2021 22:10

History, 06.12.2021 22:10






Computers and Technology, 06.12.2021 22:10

English, 06.12.2021 22:10

Mathematics, 06.12.2021 22:10

 reaction. This is a nucleophilic substitution reaction in which we have two steps. Firstly, chlorine, a good leaving group, leaves the carbon skeleton to form a relatively stable secondary carbocation. This carbocation is then attacked by the hydroxide anion, our nucleophile, to form the final product.
reaction. This is a nucleophilic substitution reaction in which we have two steps. Firstly, chlorine, a good leaving group, leaves the carbon skeleton to form a relatively stable secondary carbocation. This carbocation is then attacked by the hydroxide anion, our nucleophile, to form the final product. reaction. This is a nucleophilic substitution reaction in which we have one step. Our nucleophile, hydroxide, attacks the carbon and then chlorine leaves simultaneously without an intermediate carbocation being formed.
reaction. This is a nucleophilic substitution reaction in which we have one step. Our nucleophile, hydroxide, attacks the carbon and then chlorine leaves simultaneously without an intermediate carbocation being formed.