
Chemistry, 06.12.2021 22:10 kyliegriffis
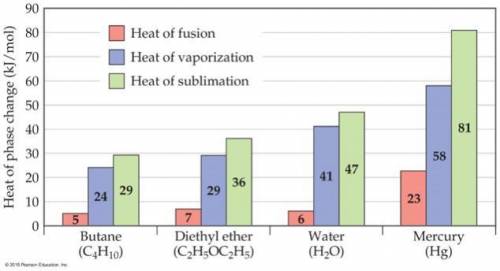
Consider the figure below. In the case of butane, diethyl ether, and water, the heat of vaporization is considerably larger than heat of fusion. Explain this phenomenon. Note that the nature of intermolecular interactions does not change through the phases because the molecules does not undergo chemical changes.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
Consider the figure below. In the case of butane, diethyl ether, and water, the heat of vaporization...
Questions

Mathematics, 20.02.2021 07:40



Mathematics, 20.02.2021 07:40


Mathematics, 20.02.2021 07:40

Chemistry, 20.02.2021 07:40

Mathematics, 20.02.2021 07:40

Mathematics, 20.02.2021 07:40




Mathematics, 20.02.2021 07:40

Computers and Technology, 20.02.2021 07:40

Mathematics, 20.02.2021 07:40

Mathematics, 20.02.2021 07:40

Mathematics, 20.02.2021 07:40


Mathematics, 20.02.2021 07:40

English, 20.02.2021 07:40



