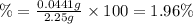The amount of nitrogen in an organic substance can be determined by an analytical method called the kjeldahl method, in which all the nitrogen in the organic substance is converted to ammonia. the ammonia, which is a weak base, can be neutralized with hydrochloric acid, as described by the equation nh 3 ( aq ) + hcl ( aq ) ⟶ nh 4 cl ( aq ) if 21.0 ml of 0.150 m hcl ( aq ) is needed to neutralize all the nh 3 ( g ) from a 2.25 g sample of organic material, calculate the mass percentage of nitrogen in the sample.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
The amount of nitrogen in an organic substance can be determined by an analytical method called the...
Questions

Mathematics, 03.01.2020 02:31



Mathematics, 03.01.2020 02:31

History, 03.01.2020 02:31



Mathematics, 03.01.2020 02:31

Geography, 03.01.2020 02:31



Mathematics, 03.01.2020 02:31



Social Studies, 03.01.2020 02:31


Mathematics, 03.01.2020 02:31



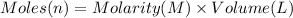
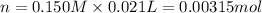
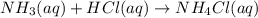
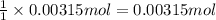 of ammonia
of ammonia