
Chemistry, 28.12.2019 05:31 carmenala2
The shielding of electrons gives rise to an effective nuclear charge, z_{\rm eff}, which explains why boron is larger than oxygen. estimate the approximate z_{\rm eff} felt by a valence electron of boron and oxygen, respectively?
a. +5 and +8
b. +3 and +6
c. +5 and +6
d. +3 and +8
e. +1 and +4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3


You know the right answer?
The shielding of electrons gives rise to an effective nuclear charge, z_{\rm eff}, which explains wh...
Questions











Mathematics, 21.01.2021 21:40


Chemistry, 21.01.2021 21:40


Chemistry, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40


Mathematics, 21.01.2021 21:40


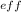 is estimated by subtracting the core electrons from the atomic number of the element.
is estimated by subtracting the core electrons from the atomic number of the element. 

