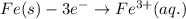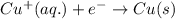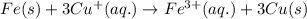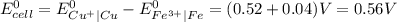Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction involving the galvanic cell made from a half-cell consisting of a copper electrode in 1 m copper(i) nitrate solution and a half-cell consisting of an iron electrode in 1 m iron(iii) nitrate. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number coefficients.) overall reaction

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

You know the right answer?
Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction invol...
Questions




Mathematics, 29.09.2019 23:30

History, 29.09.2019 23:30




Biology, 29.09.2019 23:30


Biology, 29.09.2019 23:30

Mathematics, 29.09.2019 23:30


Mathematics, 29.09.2019 23:30



History, 29.09.2019 23:30

Geography, 29.09.2019 23:30

Biology, 29.09.2019 23:30

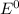 ) are given below-
) are given below-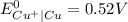
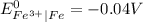
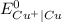 is greater than
is greater than 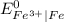 therefore Fe will be oxidized to
therefore Fe will be oxidized to 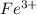 and
and 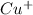 will be reduced to Cu
will be reduced to Cu