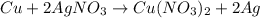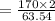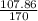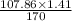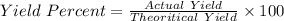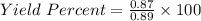Chemistry, 13.01.2020 05:31 davelopez979
In the copper – silver nitrate lab copper medals and silver nitrate solution reacted to produce silver metal and copper (ii) nitrate in solution. a student placed a copper wire with a mass of 2.93 g in the reaction test tube. the silver nitrate solution contained 1.41 g of silver nitrate. he obtained .87 g of silver metal. calculate the percent yield of silver.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
In the copper – silver nitrate lab copper medals and silver nitrate solution reacted to produce silv...
Questions


Mathematics, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30



History, 24.08.2019 22:30



Mathematics, 24.08.2019 22:30

Advanced Placement (AP), 24.08.2019 22:30


Social Studies, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30

Social Studies, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30


Mathematics, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30