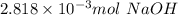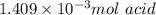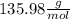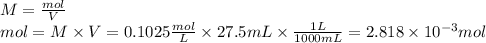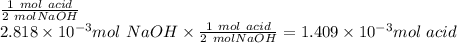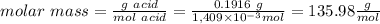Chemistry, 15.01.2020 19:31 eyeneedalife
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid was then titrated with 0.1025m naoh solution. the second equivalence point showed the sharpest change in ph, and so it was used to determine the molar mass of the unknown acid. the volume of naoh needed to reach the equivalence point was 27.5 ml.
a. calculate the number of moles of naoh used in the titration to reach the second equivalence point.
b. calculate the number of moles of diprotic acid, based on the fact that we are examining the second equivalence point.
c. calculate the molar mass of the diprotic acid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid w...
Questions




History, 23.04.2020 03:38


Mathematics, 23.04.2020 03:38

Social Studies, 23.04.2020 03:38

Health, 23.04.2020 03:38

English, 23.04.2020 03:38




Mathematics, 23.04.2020 03:38

Mathematics, 23.04.2020 03:38



Mathematics, 23.04.2020 03:38


English, 23.04.2020 03:38