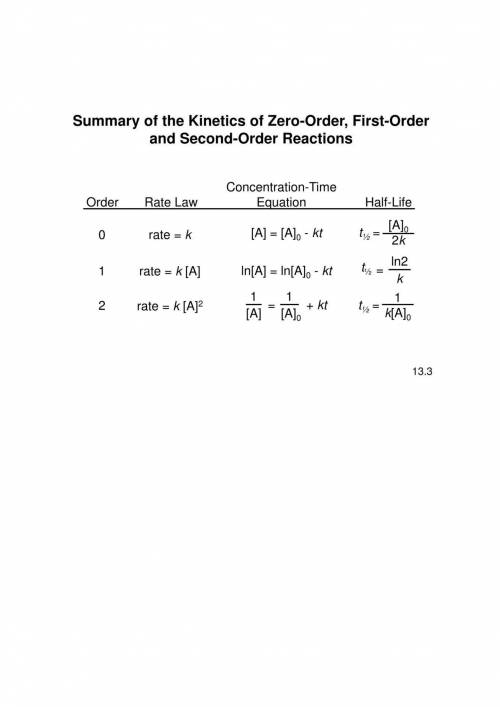
The gas-phase decomposition of ch3cho (g) occurs according to the equation
ch3cho (g) > ch...

The gas-phase decomposition of ch3cho (g) occurs according to the equation
ch3cho (g) > ch4 (g) +co (g) and is second order. the value of the rate constant is 0.105 m-1 x s-1 at 490 degrees celcius. if the concentration of ch3cho (g) is 0.012 m initially, what will be its concentration 5.0 minutes later.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Questions

English, 05.05.2020 08:57

Business, 05.05.2020 08:57



Mathematics, 05.05.2020 08:57

Chemistry, 05.05.2020 08:57

Mathematics, 05.05.2020 08:57


Mathematics, 05.05.2020 08:58




Mathematics, 05.05.2020 08:58






Mathematics, 05.05.2020 08:58

Social Studies, 05.05.2020 08:58

![[A] =8.71\times 10^{-3}](/tpl/images/0457/3112/8b81b.png) M or 0.00871 M
M or 0.00871 M![\frac{1}{[A]}=\frac{1}{[A_{0}]}+kt](/tpl/images/0457/3112/b721a.png)
![[A_{0}]](/tpl/images/0457/3112/747e3.png) = Initial concentration
= Initial concentration![\frac{1}{[A]}=\frac{1}{0.012}+ 0.105\times 300](/tpl/images/0457/3112/e46ed.png)
![\frac{1}{[A]}=83.33+ 31.5](/tpl/images/0457/3112/c3da5.png)
![\frac{1}{[A]}=114.83](/tpl/images/0457/3112/63efb.png)
![[A] =\frac{1}{114.83}](/tpl/images/0457/3112/33594.png)