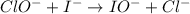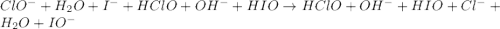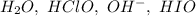Consider this mechanism:
step 1: clo-(aq) + h2o(l) hclo(aq) + oh-(aq) (fast)
step 2: i-(aq) + hclo(aq) hio(aq) + cl-(aq) (slow)
step 3: oh-(aq) + hio(aq) h2o(l) + io-(aq) (fast)
what is the overall reaction?
a.
hclo (aq) + h2o (l) oh- (aq) + cl- (aq)
b.
i- (aq) + hclo (aq) hio (aq) + cl- (aq)
c.
oh- (aq) +hio (aq) h2o (l) + io- (aq)
d.
clo- (aq) + i- (aq) cl- (aq) + io- (aq)
e.
clo- (aq) + h2o(l) hclo(aq) + oh- (aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Consider this mechanism:
step 1: clo-(aq) + h2o(l) hclo(aq) + oh-(aq) (fast)
step 2:...
step 1: clo-(aq) + h2o(l) hclo(aq) + oh-(aq) (fast)
step 2:...
Questions


Mathematics, 23.05.2020 21:02







Biology, 23.05.2020 21:02


Mathematics, 23.05.2020 21:02



English, 23.05.2020 21:02



Mathematics, 23.05.2020 21:02

Mathematics, 23.05.2020 21:02