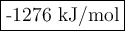()
the equation for the reaction can be shown as:
hh
h-c-0-0-h
+ 30=
...

Chemistry, 19.01.2020 17:31 brooklyn4682
()
the equation for the reaction can be shown as:
hh
h-c-0-0-h
+ 30=
0
2
0=c=o
+ 3h-o-h
hh
bond
bond energy in
kj per mole
413
347
c-h
c-c
c-o
c=0
o-h
o=0
358
799
467
495
use the bond energies to calculate the overall energy change for this
reaction.
overall energy change =


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
Questions


Mathematics, 28.09.2019 22:30

English, 28.09.2019 22:30



English, 28.09.2019 22:30


Mathematics, 28.09.2019 22:30

Biology, 28.09.2019 22:30

Arts, 28.09.2019 22:30

Chemistry, 28.09.2019 22:30

Mathematics, 28.09.2019 22:30

Mathematics, 28.09.2019 22:30




Mathematics, 28.09.2019 22:30