The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → c...

Chemistry, 20.01.2020 03:31 tatianaflores9040
The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → cacl_2 (aq) + h_2 (g)
if 500.0 g of calcium are added to an excess of hci, what volume of h_2 is produced?
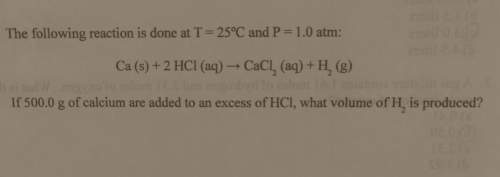

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
Questions



History, 23.10.2019 22:30












Mathematics, 23.10.2019 22:50



Chemistry, 23.10.2019 22:50


Chemistry, 23.10.2019 22:50




