
Chemistry, 23.01.2020 17:31 maisymooch
Calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 9.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2
You know the right answer?
Calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 9.0...
Questions


Mathematics, 14.07.2019 06:20


Mathematics, 14.07.2019 06:20


Mathematics, 14.07.2019 06:20

English, 14.07.2019 06:20

Mathematics, 14.07.2019 06:20


English, 14.07.2019 06:20


Mathematics, 14.07.2019 06:20


Biology, 14.07.2019 06:20


Computers and Technology, 14.07.2019 06:20


Biology, 14.07.2019 06:20

Biology, 14.07.2019 06:20


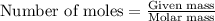
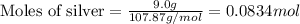
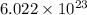 number of particles
number of particles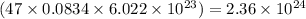 number of electrons
number of electrons


