
Chemistry, 28.01.2020 20:51 queenkimm26
Assume that each atom is a sphere, and that the surface of each atom is in contact with its nearest neighbor. determine the percentage of unit cell volume that is occupied in (a) a face- centered cubic lattice, (b) a body-centered cubic lattice, and (c) a diamond lattice.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
Assume that each atom is a sphere, and that the surface of each atom is in contact with its nearest...
Questions


Mathematics, 12.12.2019 04:31

Mathematics, 12.12.2019 04:31


Health, 12.12.2019 04:31

Biology, 12.12.2019 04:31


Mathematics, 12.12.2019 04:31




History, 12.12.2019 04:31


Computers and Technology, 12.12.2019 04:31



Mathematics, 12.12.2019 04:31



Mathematics, 12.12.2019 04:31

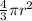
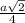
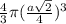 =
= 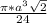
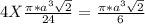
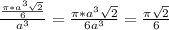 = 0.7405
= 0.7405 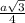
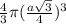 =
=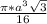
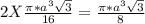
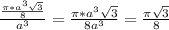 = 0.6803
= 0.6803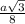
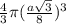 =
= 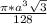
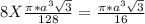
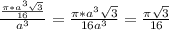 = 0.3401
= 0.3401

