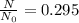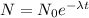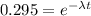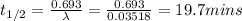Chemistry, 10.02.2020 20:25 kelsotay623
What is the half-life of bismuth-214 if 34.7 minutes are required for the mass of a sample of bismuth-214 to fall to 29.5 percent of its original value? Since the decomposition is a radioactive decay reaction, it is first order.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
What is the half-life of bismuth-214 if 34.7 minutes are required for the mass of a sample of bismut...
Questions












History, 28.06.2019 05:40

History, 28.06.2019 05:40

Chemistry, 28.06.2019 05:40


Mathematics, 28.06.2019 05:40

Computers and Technology, 28.06.2019 05:40

History, 28.06.2019 05:40