
Chemistry, 13.02.2020 19:57 CrusaderLord
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibrium in favor of products. b. Addition of SCN-(aq) will shift the equilibrium in favor of reactants. c. Addition of Cl-(aq) will shift the equilibrium in favor of reactants.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3


Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibri...
Questions


Mathematics, 12.10.2020 20:01




Computers and Technology, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01






Mathematics, 12.10.2020 20:01

Spanish, 12.10.2020 20:01

English, 12.10.2020 20:01



Mathematics, 12.10.2020 20:01


Mathematics, 12.10.2020 20:01

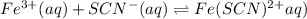
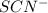 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

