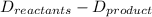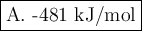Chemistry, 13.02.2020 20:53 camcollins00
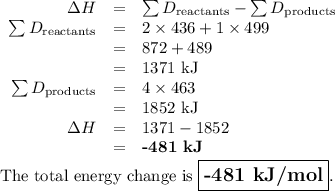
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:
H-H bond: 436 kJ/mol
O-O double bond: 499 kJ/mol
H-O bond: 463 kJ/mol
A. -481 kJ/mol
B. + 445 kJ/mol
C. +63 kJ/mol
D. -730.5 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:...
Given:...
Questions


Mathematics, 04.11.2020 18:30



Physics, 04.11.2020 18:30

Biology, 04.11.2020 18:30


Mathematics, 04.11.2020 18:30

Mathematics, 04.11.2020 18:30



Biology, 04.11.2020 18:30




Business, 04.11.2020 18:30


Social Studies, 04.11.2020 18:30


 = ( A ) ; - 481 kJ/mol
= ( A ) ; - 481 kJ/mol