
A solution of HNO 3 is standardized by reaction with pure sodium carbonate. 2 H + + Na 2 CO 3 ⟶ 2 Na + + H 2 O + CO 2 A volume of 26.66 ± 0.06 mL of HNO 3 solution was required for complete reaction with 0.9479 ± 0.0007 g of Na 2 CO 3 , (FM 105.988 ± 0.001 g/mol ). Find the molarity of the HNO 3 solution and its absolute uncertainty. Note: Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

You know the right answer?
A solution of HNO 3 is standardized by reaction with pure sodium carbonate. 2 H + + Na 2 CO 3 ⟶ 2 Na...
Questions



History, 22.07.2019 02:30





Mathematics, 22.07.2019 02:30



Mathematics, 22.07.2019 02:30





Business, 22.07.2019 02:30

Biology, 22.07.2019 02:30

Social Studies, 22.07.2019 02:30


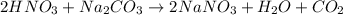
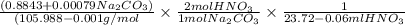
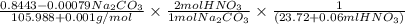
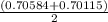 = 0.703495
= 0.703495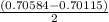
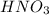 .
.

