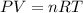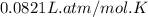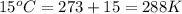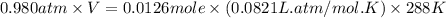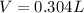Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
What volume of F2 (in liters) is required to react with 1.00 g of uranium according to the equation...
Questions

English, 28.08.2020 02:01

Computers and Technology, 28.08.2020 02:01



Mathematics, 28.08.2020 02:01

English, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01



Mathematics, 28.08.2020 02:01



Mathematics, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01


Mathematics, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01

World Languages, 28.08.2020 02:01

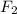 required is, 0.304 L
required is, 0.304 L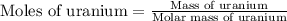
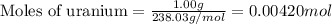
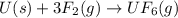
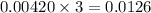 moles of
moles of