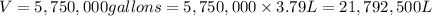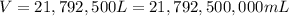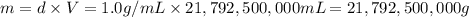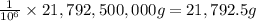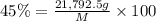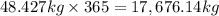Chemistry, 18.02.2020 05:36 reginagotredhair
Fluoridation is the process of adding fluorine compounds to drinking water to help fight tooth decay. A concentration of 1 ppm of fluorine is sufficient for the purpose (1 ppm means one part per million, or 1 g of fluorine per 1 million g of water). The compound normally chosen for fluoridation is sodium fluoride, which is also added to some toothpastes. Calculate the quantity of sodium fluoride in kilograms needed per year for a city of 50,000 people if the daily consumption of water per person is 115.0 gallons. (Sodium fluoride is 45.0 percent fluorine by mass. 1 gallon = 3.79 L; 1 ton=2000lb; 1 lb= 453.6 g; density of water =1.0 g/mL)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


You know the right answer?
Fluoridation is the process of adding fluorine compounds to drinking water to help fight tooth decay...
Questions


Mathematics, 17.07.2019 02:30



Mathematics, 17.07.2019 02:30



Mathematics, 17.07.2019 02:30


Mathematics, 17.07.2019 02:30




Physics, 17.07.2019 02:30

Mathematics, 17.07.2019 02:30

Mathematics, 17.07.2019 02:30




English, 17.07.2019 02:30