
Chemistry, 18.02.2020 19:53 hannahking1869
The 116-g sample was heated to 94.5°C and placed into a calorimeter containing 72 g of water at 20.0°C. The heat capacity of the calorimeter was 14.7 J/K. The final temperature in the calorimeter was 25.6°C. What is the specific heat capacity (in J/g°C) of the mineral?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
The 116-g sample was heated to 94.5°C and placed into a calorimeter containing 72 g of water at 20.0...
Questions

Social Studies, 21.10.2019 19:50


English, 21.10.2019 19:50




English, 21.10.2019 19:50



Mathematics, 21.10.2019 19:50


Mathematics, 21.10.2019 19:50

Arts, 21.10.2019 19:50


English, 21.10.2019 19:50


Mathematics, 21.10.2019 19:50

Mathematics, 21.10.2019 19:50


Mathematics, 21.10.2019 19:50

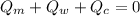
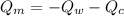 ----------------equation (1)
----------------equation (1)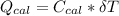 ---------------- equation (2)
---------------- equation (2)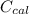 = heat capacity of the calorimeter
= heat capacity of the calorimeter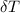 = change in temperature
= change in temperature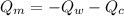
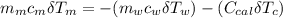
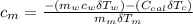 ---------------- equation (4)
---------------- equation (4)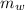 ) = 72g
) = 72g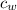 ) = 4.18 J/g.k
) = 4.18 J/g.k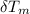 ) = 25.6 °C - 94.5 °C
) = 25.6 °C - 94.5 °C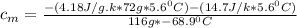
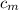 = 0.221172 J/g°C
= 0.221172 J/g°C

