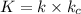Chemistry, 19.02.2020 01:25 NetherisIsTheQueen
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromine.
step 1 fast: NO Br2 NOBr2
step 2 slow: NOBr2 NO 2 NOBr
(1) What is the equation for the overall reaction
(2) Enter the formula of any species that acts as a reaction intermediate?
(3) Complete the rate law for the overall reaction that is consistent with this mechanism.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 15:00
How much more basic is a solution with ph 8 than a solution with ph 7
Answers: 1
You know the right answer?
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromi...
Questions


Mathematics, 25.05.2021 02:20

Mathematics, 25.05.2021 02:20

Mathematics, 25.05.2021 02:20






English, 25.05.2021 02:20


Mathematics, 25.05.2021 02:20

Mathematics, 25.05.2021 02:20

English, 25.05.2021 02:20


English, 25.05.2021 02:20

Biology, 25.05.2021 02:20


Arts, 25.05.2021 02:20

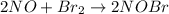
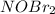 .
.![R=K[NO]^2[Br]](/tpl/images/0515/0512/945c3.png)
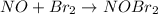 ..[1]
..[1]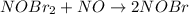 ...[2]
...[2]![R=k[NOBr_2][NO]](/tpl/images/0515/0512/7dc1f.png) ..[3]
..[3]![K_c=\frac{[NOBr_2]}{[NO][Br_2]}](/tpl/images/0515/0512/8d77b.png)
![[NOBr_2]=K_c\times [NO][Br_2]](/tpl/images/0515/0512/df1dc.png)
![[NOBr_2]](/tpl/images/0515/0512/86582.png) rate expression [3]:
rate expression [3]:![R=k\times k_c[NO][NO][NO]=K[NO]^2[Br]](/tpl/images/0515/0512/f99df.png)