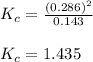Chemistry, 19.02.2020 01:51 bougiehairstudios
When 9.2 g of frozen N2O4 is added to a 0.50 L reaction vessel and the vessel is heated to 400 K and allowed to come to equilibrium, the concentration of N2O4 is determined to be 0.057 M. Given this information, what is the value of Kc for the reaction below at
400 K? N2O4(g) ⇌ 2 NO2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

You know the right answer?
When 9.2 g of frozen N2O4 is added to a 0.50 L reaction vessel and the vessel is heated to 400 K and...
Questions

Mathematics, 21.01.2020 23:31


English, 21.01.2020 23:31


History, 21.01.2020 23:31

Health, 21.01.2020 23:31

History, 21.01.2020 23:31





Biology, 21.01.2020 23:31

Mathematics, 21.01.2020 23:31

Chemistry, 21.01.2020 23:31



Mathematics, 21.01.2020 23:31



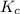 for the given reaction is 1.435
for the given reaction is 1.435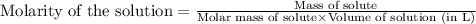
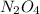 = 9.2 g
= 9.2 g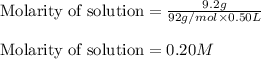
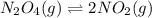
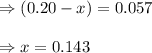
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0515/1174/271f5.png)
![[NO_2]_{eq}=2x=(2\times 0.143)=0.286M](/tpl/images/0515/1174/a94f2.png)
![[N_2O_4]_{eq}=0.057M](/tpl/images/0515/1174/d44e5.png)