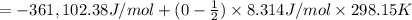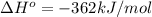A sample of K(s) of mass 2.720 g undergoes combustion in a constant volume calorimeter at 298.15 K. The calorimeter constant is 1849 J K−1, and the measured temperature rise in the inner water bath containing 1439 g of water is 1.60 K.
Part A
Calculate ΔU∘f for K2O.
Express your answer to three significant figures and include the appropriate units.
Part B
Calculate ΔH∘f for K2O.
Express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
A sample of K(s) of mass 2.720 g undergoes combustion in a constant volume calorimeter at 298.15 K....
Questions


History, 20.02.2020 03:32


Biology, 20.02.2020 03:32


History, 20.02.2020 03:32

Biology, 20.02.2020 03:32


Computers and Technology, 20.02.2020 03:32









Mathematics, 20.02.2020 03:32


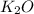 is -361 kJ/mol.
is -361 kJ/mol.![q=[q_1+q_2]](/tpl/images/0516/2162/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0516/2162/1d50b.png)
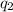 = heat absorbed by the water
= heat absorbed by the water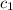 = specific heat of calorimeter =
= specific heat of calorimeter = 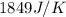
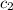 = specific heat of water =
= specific heat of water = 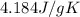
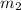 = mass of water = 1439 g
= mass of water = 1439 g = change in temperature =
= change in temperature = 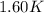
![q=[(1849 J/K \times 1.60 K)+(1439 g \times 4.184J/gK\times 1.60 K)]](/tpl/images/0516/2162/36fc3.png)
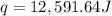
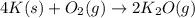
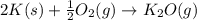
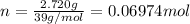
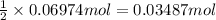 of
of 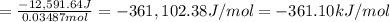
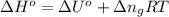
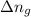 = moles of gases on RHS - moles of gasses on LHS
= moles of gases on RHS - moles of gasses on LHS