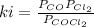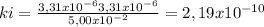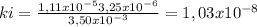Chemistry, 20.02.2020 05:59 kayranicole1
At 100oC the equilibrium constant for the reaction below has the value of Keq = 2.19 x 10−10. COCl2(g) ⇄ CO(g) + Cl2(g) Are the following mixtures of COCl2, CO, and Cl2 at equilibrium? If not, indicate the direction that the reaction must proceed to achieve equilibrium. (i) PCOCl2 = 5.00 × 10−2 atm; PCO = 3.31 × 10−6 atm; PCl2 = 3.31 × 10−6 atm (ii) PCOCl2 = 3.50 × 10−3 atm; PCO = 1.11 × 10−5 atm; PCl2= 3.25 × 10−6 atm
(i) not at equilibrium, left to right (ii) equilibrium
(i) equilibrium, (ii) not at equilibrium, right to left
(i) equilibrium, (ii) not at equilibrium, left to right
(i) not at equilibrium, right to left, (ii) equilibrium
(i) equilibrium, (ii) equilibrium

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
At 100oC the equilibrium constant for the reaction below has the value of Keq = 2.19 x 10−10. COCl2(...
Questions

English, 02.09.2020 18:01


English, 02.09.2020 18:01

Advanced Placement (AP), 02.09.2020 18:01

History, 02.09.2020 18:01




English, 02.09.2020 18:01





Mathematics, 02.09.2020 18:01


Biology, 02.09.2020 18:01

Advanced Placement (AP), 02.09.2020 18:01