Chemistry, 20.02.2020 22:01 bartlettcs9817
Reaction is found to have a rate constant of 12.5 s-1 at 25.0 Celsius. When you heat the reaction up by ten degrees Celsius, the rate of the reaction exactly doubles. What is the activation energy for this reaction in kJ/mol?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Reaction is found to have a rate constant of 12.5 s-1 at 25.0 Celsius. When you heat the reaction up...
Questions

Mathematics, 04.02.2021 01:40


Advanced Placement (AP), 04.02.2021 01:40

Geography, 04.02.2021 01:40

Mathematics, 04.02.2021 01:40


Mathematics, 04.02.2021 01:40

Spanish, 04.02.2021 01:40




History, 04.02.2021 01:40

Mathematics, 04.02.2021 01:40

Social Studies, 04.02.2021 01:40

Health, 04.02.2021 01:40

Mathematics, 04.02.2021 01:40

Mathematics, 04.02.2021 01:40

Mathematics, 04.02.2021 01:40

Mathematics, 04.02.2021 01:40

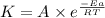
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0517/9661/6d953.png)
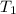 = initial temperature =
= initial temperature = 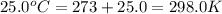
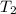 = final temperature =
= final temperature = 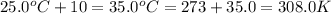
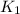 = rate constant at
= rate constant at 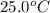 =
= 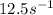
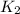 = rate constant at
= rate constant at 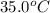 =
= 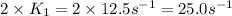
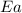 = activation energy for the reaction = ?
= activation energy for the reaction = ?![\log (\frac{25.0s^{-1}}{12.5s^{-1}})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{298.0K}-\frac{1}{308.0K}]](/tpl/images/0517/9661/6e305.png)