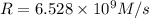Chemistry, 21.02.2020 00:00 ylianafghgfdsnm1479
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way:
OCl−+I−→OI−+Cl−.
This rapid reaction gives the following rate data:
[OCl−](M) [I]−(M) Rate (M/s)
1.5×10^−3 1.5×10^−3 1.36×10^−4
3.0×10^−3 1.5×10^−3 2.72×10^−4
1.5×10^−3 3.0×10^−3 2.72×10^−4
a. Write the rate law for this reaction.
b. Calculate the rate constant with proper units.
c. Calculate the rate when [OCl-]= 1.8×10^3 M and [I-]= 6.0×10^4 M .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the foll...
Questions



Social Studies, 11.03.2020 22:53




Computers and Technology, 11.03.2020 22:53









Computers and Technology, 11.03.2020 22:53

Geography, 11.03.2020 22:53


Computers and Technology, 11.03.2020 22:53

World Languages, 11.03.2020 22:53

![R=k[OCl^-]^1\times [I^-]^1](/tpl/images/0518/3250/d583c.png)
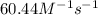 .
.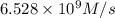 .
.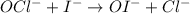
![R=k[OCl^-]^x\times [I^-]^y](/tpl/images/0518/3250/04fdb.png)
![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/e45d8.png) ..[1]
..[1]![[OCl^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/3317d.png) and
and ![2.72\times 10^{-4}M/s=k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/dea0f.png) ..[2]
..[2]![[I^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/b24ad.png) .
.![2.72\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y](/tpl/images/0518/3250/22116.png) ..[3]
..[3]![\frac{1.36\times 10^{-4}M/s}{2.72\times 10^{-4}M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}](/tpl/images/0518/3250/ebd3d.png)
![\frac{1.36\times 10^{-4} M/s}{2.72\times 10^{-4} M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y}](/tpl/images/0518/3250/11fc2.png)
![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]](/tpl/images/0518/3250/f3ed7.png)
![k=\frac{1.36\times 10^{-4} M/s}{[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]}=60.44 M^{-1}s^{-1}](/tpl/images/0518/3250/1ed07.png)
![[OCl^-]=1.8\times 10^{3} M](/tpl/images/0518/3250/b9482.png) and
and ![[I^-]=6.0\times 10^{4} M](/tpl/images/0518/3250/ceced.png) be R.
be R.![R=60.44 M^{-1}s^{-1}\times [1.8\times 10^{3} M]^1\times [6.0\times 10^{4} M]^1](/tpl/images/0518/3250/1384b.png)