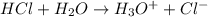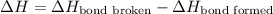Chemistry, 21.02.2020 02:31 chanelandme123
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking any bond is an endothermic process and making any bond is exothermic, what is the net enthalpy change involved in the reaction between HI and H2O? Is this reaction endothermic or exothermic?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2


Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3
You know the right answer?
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking an...
Questions


Arts, 28.09.2020 09:01





English, 28.09.2020 09:01


Mathematics, 28.09.2020 09:01


Business, 28.09.2020 09:01




Mathematics, 28.09.2020 09:01





Mathematics, 28.09.2020 09:01