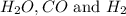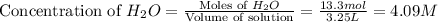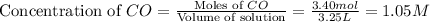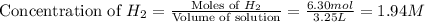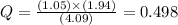The following reaction was carried out in a 3.25 L reaction vessel at 1100 K:
If during...

The following reaction was carried out in a 3.25 L reaction vessel at 1100 K:
If during the course of the reaction, the vessel is found to contain 5.25 mol of C, 13.3 mol of H₂O, 3.40 mol of CO, and 6.30 mol of H₂, what is the reaction quotient Q?
Enter the reaction quotient numerically.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Questions

Medicine, 16.04.2020 19:24

Mathematics, 16.04.2020 19:24




Social Studies, 16.04.2020 19:24


Mathematics, 16.04.2020 19:24


Mathematics, 16.04.2020 19:25




Mathematics, 16.04.2020 19:25

Physics, 16.04.2020 19:25





Mathematics, 16.04.2020 19:25

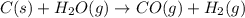
![Q=\frac{[CO][H_2]}{[H_2O]}](/tpl/images/0519/6055/f765a.png)