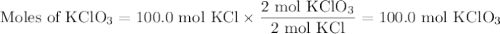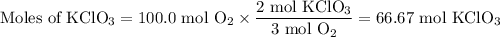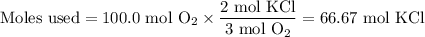Chemistry, 24.02.2020 07:21 DASASDAEDWEDA
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of KCl and 100.0 moles of O2...
what is the limiting reactant? (TYPE EITHER KCl or O2)
what is the excess reactant? (TYPE EITHER KCl or O2)
how many moles of the excess reactant will be left over? moles (TYPE just the number to the correct amount of significant figures)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3


Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of...
Questions




English, 12.02.2020 22:49








History, 12.02.2020 22:49


Mathematics, 12.02.2020 22:49

Health, 12.02.2020 22:49

Social Studies, 12.02.2020 22:49

Geography, 12.02.2020 22:49

Mathematics, 12.02.2020 22:49