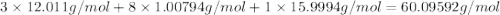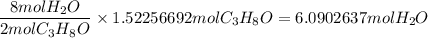Chemistry, 24.02.2020 07:43 bridgettebach
When 91.5 g of isopropyl alcohol which has an empirical formula of C3H8O is burned in excess oxygen gas, how many grams of H2O are formed? MWC = 12.011 g/mol, MWH = 1.00794 g/mol, and MWO = 15.9994 g/mol.
1. 47.9229
2. 84.1255
3. 52.2617
4. 49.8948
5. 86.9152
6. 119.705
7. 88.0758
8. 76.2076
9. 62.9663
10. 109.729

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
When 91.5 g of isopropyl alcohol which has an empirical formula of C3H8O is burned in excess oxygen...
Questions

English, 11.11.2019 06:31




Mathematics, 11.11.2019 06:31

Biology, 11.11.2019 06:31








Health, 11.11.2019 06:31

English, 11.11.2019 06:31

Computers and Technology, 11.11.2019 06:31

History, 11.11.2019 06:31

Business, 11.11.2019 06:31

Social Studies, 11.11.2019 06:31

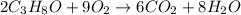
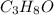 produce 8 moles of
produce 8 moles of 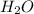 .
.