
Chemistry, 25.02.2020 03:23 tdyson3p6xvtu
Calculate the standard heat of reaction for the following methane-generating reaction of methanogenic bacteria: 4CH3NH2(g) + 2H2O(l) → 3CH4(g) + CO2(g) + 4NH3(g) Given that ΔHfo(CH3NH2, g) = –22.97 kJ/mol; ΔHfo(H2O, l) = –285.8 kJ/mol; ΔHfo(CH4, g) = –74.8 kJ/mol; ΔHfo(CO2, g) = –393.5 kJ/mol ΔHfo(NH3, g) = –46.1 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Calculate the standard heat of reaction for the following methane-generating reaction of methanogeni...
Questions

Health, 31.07.2019 05:00

Geography, 31.07.2019 05:00

Mathematics, 31.07.2019 05:00




Social Studies, 31.07.2019 05:00




Social Studies, 31.07.2019 05:00




History, 31.07.2019 05:00

Geography, 31.07.2019 05:00




Biology, 31.07.2019 05:00

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0522/6322/e893d.png)
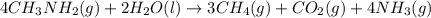
![\Delta H_{rxn}=[(3\times \Delta H_f_{(CH_4(g))})+(1\times \Delta H_f_{(CO_2(g))})+(4\times \Delta H_f_{(NH_3(g))})]-[(4\times \Delta H_f_{(CH_3NH_2(g))})+(2\times \Delta H_f_{(H_2O(l))})]](/tpl/images/0522/6322/6730f.png)
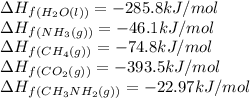
![\Delta H_{rxn}=[(3\times (-74.8))+(1\times (-393.5))+(4\times (-46.1))]-[(4\times (-22.97))+(2\times (-285.8))]\\\\\Delta H_{rxn}=-138.82kJ](/tpl/images/0522/6322/fdc01.png)


