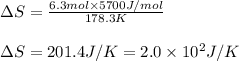Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
The heat of fusion of acetone is 5.7 kJ/mol. Calculate to two significant figures the entropy change...
Questions

Mathematics, 06.05.2020 01:59



Mathematics, 06.05.2020 01:59


English, 06.05.2020 01:59

Mathematics, 06.05.2020 01:59

Mathematics, 06.05.2020 01:59

Mathematics, 06.05.2020 01:59


Advanced Placement (AP), 06.05.2020 01:59


Mathematics, 06.05.2020 01:59


Chemistry, 06.05.2020 01:59

History, 06.05.2020 01:59

History, 06.05.2020 01:59

History, 06.05.2020 01:59

Mathematics, 06.05.2020 01:59

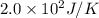
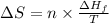
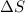 = Entropy change
= Entropy change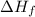 = enthalpy of fusion = 5.7 kJ/mol = 5700 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 5.7 kJ/mol = 5700 J/mol (Conversion factor: 1 kJ = 1000 J)![-94.7^oC=[273-94.7]=178.3K](/tpl/images/0522/9891/25895.png)