Chemistry, 25.02.2020 19:47 kaylailkanic1487
Be sure to answer all parts. For the titration of 10.0 mL of 0.250 M acetic acid with 0.200 M sodium hydroxide, determine the pH when: (a) 10.0 mL of base has been added. (b) 12.5 mL of base has been added. (c) 15.0 mL of base has been added.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Be sure to answer all parts. For the titration of 10.0 mL of 0.250 M acetic acid with 0.200 M sodium...
Questions

Chemistry, 09.01.2020 06:31



Chemistry, 09.01.2020 06:31

Chemistry, 09.01.2020 06:31




Chemistry, 09.01.2020 06:31





Chemistry, 09.01.2020 06:31



Chemistry, 09.01.2020 06:31

Chemistry, 09.01.2020 06:31


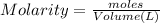
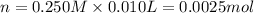
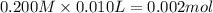
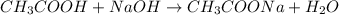
![[H^+]=\frac{0.0005 mol}{0.020 L}=0.025 M](/tpl/images/0523/5054/d4db0.png)
![pH=-\log[H^+]=-\log[0.025 M]=1.60](/tpl/images/0523/5054/f1f2d.png)
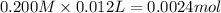
![[H^+]=\frac{0.0001 mol}{0.022 L}=0.0045 M](/tpl/images/0523/5054/5492d.png)
![pH=-\log[H^+]=-\log[0.0045 M]=2.34](/tpl/images/0523/5054/d5f92.png)
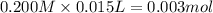
![[OH^-]=\frac{0.0005 mol}{0.025 L}=0.02 M](/tpl/images/0523/5054/d1bd6.png)
![pOH=-\log[OH^-]=-\log[0.02 M]=1.70](/tpl/images/0523/5054/8bd06.png)