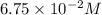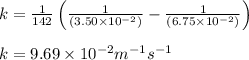Chemistry, 26.02.2020 05:43 Jadaflournoy5
Hydrogen iodide decomposes at 800 K via a second-order process to produce hydrogen and iodine according to the following chemical equation. 2 HI(g) ---> H2 (g) + I2 g) At 800 K it takes 142 seconds for the initial concentration of HI to decrease from 6.75 x 10^-2 M to 3.50 x 10^-2 M. What is the rate constant for the reaction at this temperature?
A) 5.12 x 10^-4 M^-1 s^-1
B) 9.69 x 10^-2 M^-1 s^-1
C) 10.3 M^-1 s^-1
D) 1.95 x 10^3 M^-1 s^-1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
Hydrogen iodide decomposes at 800 K via a second-order process to produce hydrogen and iodine accord...
Questions





History, 15.07.2021 23:10




English, 15.07.2021 23:10


History, 15.07.2021 23:10


Mathematics, 15.07.2021 23:10

Mathematics, 15.07.2021 23:10


Mathematics, 15.07.2021 23:10




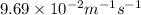
![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0524/6600/5ea71.png)
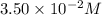
![[A]_o](/tpl/images/0524/6600/9caf5.png) = Initial concentration =
= Initial concentration =