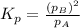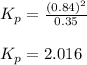Chemistry, 27.02.2020 19:50 superfly903
Consider the hypothetical reaction A(g)←→2B(g). A flask is charged with 0.77 atm of pure A, after which it is allowed to reach equilibrium at 0 ∘C. At equilibrium the partial pressure of A is 0.35 atm .A: What is the total pressure in the flask at equilibrium?
B:What is the value of Kp?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1


Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Consider the hypothetical reaction A(g)←→2B(g). A flask is charged with 0.77 atm of pure A, after wh...
Questions


Advanced Placement (AP), 26.06.2020 15:01

Mathematics, 26.06.2020 15:01


History, 26.06.2020 15:01

Geography, 26.06.2020 15:01




Mathematics, 26.06.2020 15:01

Health, 26.06.2020 15:01





History, 26.06.2020 15:01


Mathematics, 26.06.2020 15:01

English, 26.06.2020 15:01

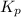 for the given equation is 2.016
for the given equation is 2.016
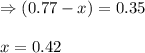
![p^A_{eq}+p^b_{eq}=[0.35+0.84]atm=1.19atm](/tpl/images/0527/0172/9c43a.png)