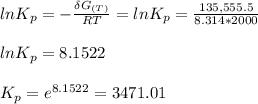Chemistry, 28.02.2020 02:03 Lizzy527663
Calculate the equilibrium composition for the reaction H2−− 1 2O2 −−)−−* H2O when the ratio of the number of moles of elemental hydrogen to elemental oxygen is unity. Perform this calculation at T = 2000 K and P = 1 atm.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1


Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

You know the right answer?
Calculate the equilibrium composition for the reaction H2−− 1 2O2 −−)−−* H2O when the ratio of the n...
Questions

Mathematics, 22.11.2019 06:31

History, 22.11.2019 06:31

Mathematics, 22.11.2019 06:31



Mathematics, 22.11.2019 06:31

Physics, 22.11.2019 06:31


English, 22.11.2019 06:31





Biology, 22.11.2019 06:31

Mathematics, 22.11.2019 06:31

English, 22.11.2019 06:31

Mathematics, 22.11.2019 06:31



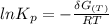 ---------(i)
---------(i)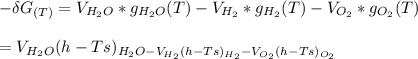
![= V_{H_20[h_f+h_{2000}-hs)-Ts]H_2O - V_{H_2}[h_f+h_{2000}-hs)-Ts]H_2- V_{O_2}[h_f+h_{2000}-hs)-Ts]O_2\\](/tpl/images/0527/5740/e8071.png)