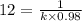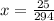Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
A second-order reaction has a half-life of 12 s when the initial concentration of reactant is 0.98 M...
Questions


History, 13.08.2019 05:10



Mathematics, 13.08.2019 05:20

Mathematics, 13.08.2019 05:20

Mathematics, 13.08.2019 05:20

Mathematics, 13.08.2019 05:20

History, 13.08.2019 05:20



Computers and Technology, 13.08.2019 05:20








Business, 13.08.2019 05:20

![[A_o]](/tpl/images/0528/9810/dc622.png) is the initial concentration = 0.98 M
is the initial concentration = 0.98 M
![t_{1/2}=\frac{1}{k[A_o]}](/tpl/images/0528/9810/4d220.png)